It’s time to move past the COVID treatment culture wars.
We’ve spent a lot of time as a society over the past two years arguing about ivermectin and hydroxychloroquine.
I know that I’ve spent way too much time and energy looking at these medications. I’ve written about both of them in this newsletter. There’s a great deep dive on ivermectin (even deeper than the one I did) in the Astral Codex newsletter if you’re interested.
My TL;DR take on these meds for COVID:
Hydroxychloroquine doesn’t seem to do much and it’s been tested extensively in lots of different populations. We gave it to everyone hospitalized early on - it didn’t do much to make anyone better and may have caused some harm
Ivermectin probably doesn’t work for COVID although the data are limited and tough to decipher because there’s concern about some fraudulent science. I think it’s possible that it has a small effect against COVID, but the data are too crappy for us to really know
But the COVID culture wars have turned these medications into flash points rather than risk/benefit discussions.
And with Omicron raging, I’m hopeful that fluvoxamine will finally be the treatment that gets us past the COVID culture wars.
I wrote about fluvoxamine early on in the pandemic when we only had one small trial on it, but there’s been important new evidence published since that time that’s led me to believe that it’s a really important part of how we should be treating COVID patients.
Fluvoxamine is everything that we wish ivermectin and hydroxychloroquine were - cheap, safe, and available - with one additional benefit: we can have confidence that it actually works in COVID
Fluvoxamine (brand name Luvox) is a selective serotonin reuptake inhibitor (SSRI) that is usually used for treatment of anxiety and depression. It’s also sometimes used for the treatment of obsessive compulsive disorder.
Fluvoxamine seems to have some anti-inflammatory effects, which is why it was considered for the treatment of COVID.
It’s been around for a while and approved for use in all populations, including children.
When a medication has been prescribed for a long time - fluvoxamine was approved in the 1990s - we can feel comfortable that we understand its side effect profile well.
Fluxiamine is well tolerated and cheap. It’s readily available and we know what to expect from its use.
So all of the issues that plague our wide deployment of other COVID treatments don’t really apply when it comes to fluvoxamine.
We don’t have to worry that we don’t have enough (like we do with monoclonal antibodies or Paxlovid). We don’t have the cost concerns that that come up with remdesivir or the mutating-the-virus concerns that come up with molnupiravir.
And fluvoxamine seems to work in COVID.
The TOGETHER trial showed that fluvoxamine reduced the risk of hospitalization compared to placebo when given early in COVID
The TOGETHER trial was a high quality clinical trial done in small hospitals across Brazil. The trial was randomized, double blinded, and placebo controlled. In other words, a gold standard clinical trial.
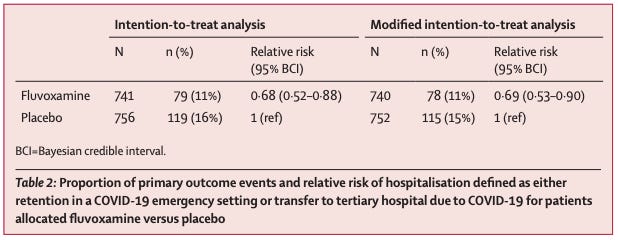
The study authors assessed the impact of fluvoxamine on whether or not patients with confirmed COVID needed to be transferred to a tertiary hospital or spend more than 6 hours in the emergency room.
In plain English: they took people who had mild COVID, gave them fluvoxamine or a placebo, and monitored to see who needed a high level of medical care.
11% of patients in the fluvoxamine group worsened enough to need more medical care versus 16% in the group given placebo:
It may not be the biggest effect size in the world, but it’s statistically significant, and the study was rigorous.
And for a medication that’s cheap, available, and well tolerated, it’s hard to argue that this is anything other than a massive win.
I think I was wrong about fluvoxamine when I first wrote about it
Fluvoxamine first came across my radar screen after a small trial published in JAMA in November of 2020 suggested that it could be beneficial as an early treatment for COVID patients.
I wrote at the time that I wasn’t persuaded by such a small trial where more patients were lost to follow up than worsened with COVID. I wanted to wait for something bigger before concluding that the benefit outweighed the risk.
But while I think I was spot on with my assessment of the benefit at that point, I think I overstated the risk of a drug that’s often used for months (if not years) being given for 10 days as a COVID treatment.
In a case like this where the risk is negligible and the cost is low, I shouldn’t have been so dismissive of the potential of fluvoxamine.
But now that we have high quality data, we should start using this drug!
It’s hard to make a case that fluvoxamine doesn’t deserve a major role in COVID treatment across the globe, particularly in those folks with high risk of progression.
I think the downside is incredibly low and there is compelling evidence that it will have a small but real impact on risk of progression.
Obviously, any discussion of a prescription drug treatment should take place in the context of a physician-patient relationship (and a newsletter doesn’t count!), but I think there’s something real here.
Now, although a short course of treatment for COVID probably isn’t long enough for fluvoxamine to have the antidepressant/anxiolytic effect that it was originally developed for, that doesn’t mean it has zero impact there.
Some would advocate for tapering rather than just stopping a drug like this even after a short course; tapering probably deserves a place in any individual treatment discussion, but the trial protocols didn’t include a tapering strategy.
So hopefully fluvoxamine is a step past the COVID treatment culture wars towards a place where we can talk about risks and benefits for each individual rather than debate the merits of whatever Joe Rogan may have been prescribed.