Do you ever learn about something and wonder why it isn’t a way bigger deal?
That’s how I feel about CRISPR technology and the genetic engineering revolution that it seems to promise.
Last October, I wrote about Jennifer Doudna and Emmanuelle Charpentier winning the Nobel Prize “for the development of a method for genome editing” because of their work on the CRISPR-Cas9 system. And since then, we’ve had some major developments in the story.
CRISPR technology provides a means to edit our DNA - so it means that people can play God in a way that we haven’t done just yet.
And while CRISPR has the potential to be a technology that revolutionizes everything from medicine to agriculture to longevity, it doesn’t get as much attention in the press as palace intrigue at the White House.
Think about it: how much have you heard about CRISPR?
And, more importantly, did you know that CRISPR is already being tested in real people with real diseases and showing really promising results?
A quick refresher on CRISPR
CRISPR technology harnesses biologic activities that were originally discovered as part of an immune system in some kinds of bacteria.
These bacteria evolved a system of cutting DNA very precisely as a way to remember viruses that infected them.
The researchers working on CRISPR tech are developing methods of harnessing the bacterial DNA slicing to make precise edits in the DNA of people.
If this technology matures the way that it’s been promised, the end result may be the ability to edit our DNA like we edit Microsoft Word documents - something so easy and quick that you don’t need much expertise to use it.
How will CRISPR be used?
CRISPR will theoretically work most effectively on traits or diseases that are impacted by a single gene. This has significant implications on how the technology might be deployed.
Think about a disease like sickle cell anemia - it’s a life threatening medical issue caused by a single genetic mutation. The simplicity of a single gene to fix makes it an incredibly promising target of CRISPR therapy. It’s certainly conceivable that we’ll see a widely deployed, safe, and effective cure of sickle cell anemia sooner rather than later.
Not every disease will be that easy to engineer away.
Disorders like high blood pressure or diabetes are going to be really tough to treat with genetic engineering since there are many different genes that impact them (and these many genes have incredibly complex interactions).
But CRISPR isn’t necessarily just a means of treating disease, there’s the potential for “enhancement” in people without disease.
One of the ethical dilemmas with this technology is that the slope between treatment and enhancement isn’t clear.
Ezra Klein and Walter Isaacson discuss this on a recent podcast: unrestricted deployment of fully mature CRISPR technology in our capitalist society may mean that rich people can increase their muscle mass or lengthen their lives, creating a massive biologic inequities that perpetuate inequality.
Think, “the future is here, it’s just not evenly distributed,” but on a genetic level.
But enough about the future, let’s talk more about the present.
The big news: CRISPR in people is already here
This may feel like science fiction, but the reality is that CRISPR is already being tested in people.
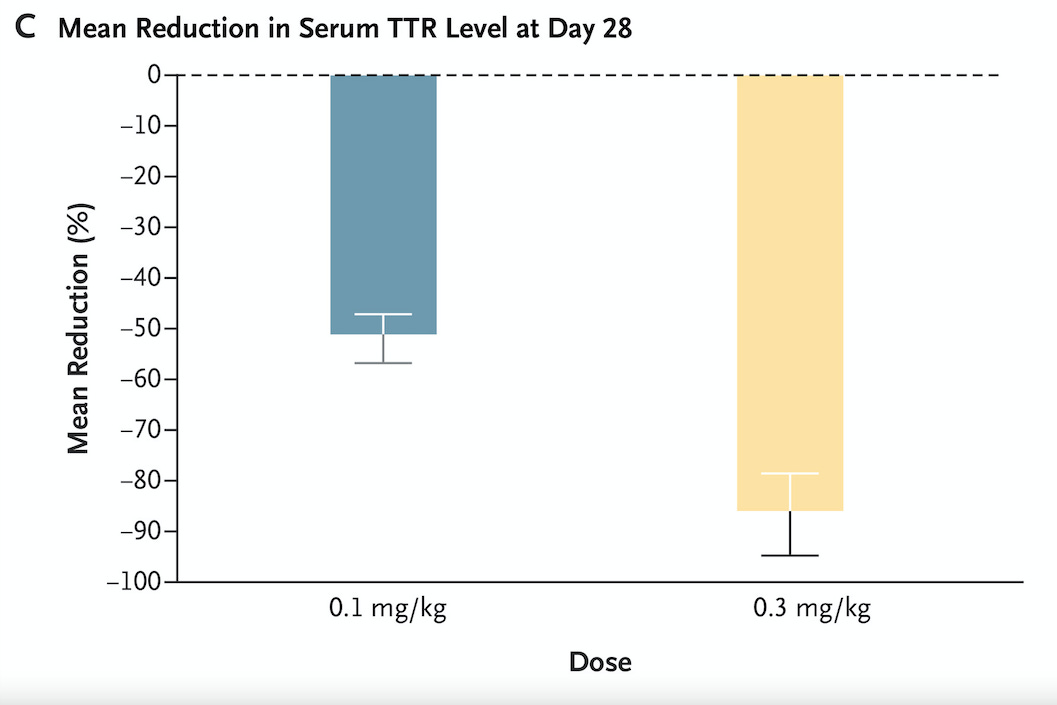
Intellia Therapeutics (a company founded by Nobel Prize winning CRISPR researcher Jennifer Doudna) just published results from a clinical trial in the New England Journal of Medicine on the use of CRISPR technology to treat a disease called TTR amyloidosis.
TTR amyloidosis is a disease where a protein called transthyretin (TTR) becomes misfolded and as a consequence gets deposited in various organs throughout the body, particularly the heart and nerves. This can result in neuropathy and congestive heart failure. People with symptomatic TTR amyloidosis have a life expectancy on the order of 2-6 years after being diagnosed with cardiac involvement.
The researchers in this study used CRISPR technology in 6 people with TTR amyloidosis and found huge reductions in blood levels of TTR that persisted out to a month (the graph below shows percent reductions in blood levels of this protein with 2 different treatment doses):
Longer follow up is planned, but it’s pretty remarkable to see an effect a month after treatment, suggesting a durable treatment.
This is crazy!
All of the patients in the study tolerated the treatment well. There was a signal of some potential for negative side effects - measures of blood coagulation rose, which makes you worry a bit about risk of blood clots, even though no negative clinical outcomes were seen (the abnormal blood tests still raise questions even though they didn’t lead to clear harm).
This is a gigantic advance of an amazing technology. But everything here is still super preliminary
This is a monumental achievement, but it’s far from evidence that we’re ready to start deploying CRISPR to all patients with TTR amyloidosis.
You worry about a handful of negative side effects that aren’t recognized in the short term of the study period:
What’s the biologic risk over the long haul from blocking the production of a protein that we evolved to make throughout our lives?
Is there any potential for off target genetic editing? CRISPR better be essentially 100% accurate before we start using it regularly.
Are there any significant side effects when this is administered to a larger population?
Does blocking production of the protein lead to improvement in the outcomes that we care about? Do these patients live longer? Do they have less heart failure? Does their neuropathy improve?
But this is only the beginning. The authors of the trial describe the potential for where this technology can lead, so I’ll just let them have the final word:
“The CRISPR-Cas9 approach used for NTLA-2001 is modular and has the capacity to be adapted to treat other diseases with simple replacement of the sgRNA. Indeed, the in vivo gene-editing approach used in this study is currently being investigated for use in other diseases. Further clinical programs involving CRISPR-Cas9–based gene-editing strategies are planned by many investigators for a wide range of diseases; these programs may make use of the potential not only to knock out expression of harmful protein products but also to insert genes to produce functional proteins where mutations cause pathologic deficiencies.”
Thank you for reading! Please share on social media and encourage your friends and family to subscribe!