Is the Moderna vaccine better than the Pfizer vaccine?
Which one should you choose if you have an option?
The Pfizer COVID vaccine is a home run. And it seems that the Moderna vaccine is too.
Moderna and the FDA release the 54 page briefing document with data on efficacy, side effects, and the study population. The document has a lot of information so we can learn quite a bit about this vaccine.
Let’s take a look at the Moderna vaccine and then compare it to the one from Pfizer/BioNTech.
As with Pfizer trial, I think that there’s value from a detailed dive into the data to unpack more information about efficacy and safety.
The trial design really matters, both in terms of who was included and how they evaluated efficacy.
Who were the patients in this trial?
This was a trial looking at 27,817 patients over age 18. 13,934 patients received the vaccine and 13,883 patients received the placebo. This vaccine is administered in 2 doses 28 days apart (contrasted with 21 days apart for the Pfizer vaccine).
About half the participants were women.
Median age was 51, and 75% were between 18-65 years old with the remaining quarter over age 65.
About 80% were white, about 10% Black, about 20% Hispanic (just like with Pfizer vaccine, there’s an overlap between white and Hispanic). Under 5% were Asian.
The briefing report alternatively reports the race/ethnicity breakdown as 63.3% non-Hispanic white and 36.5% communities of color.
This was a healthy group - almost 80% had no high risk conditions and only 4% had 2 or more high risk conditions, which were defined as:
Chronic lung disease
Significant cardiac disease
Severe obesity (BMI ≥40)
Diabetes
Liver disease
HIV infection
How effective is this vaccine?
Really effective!
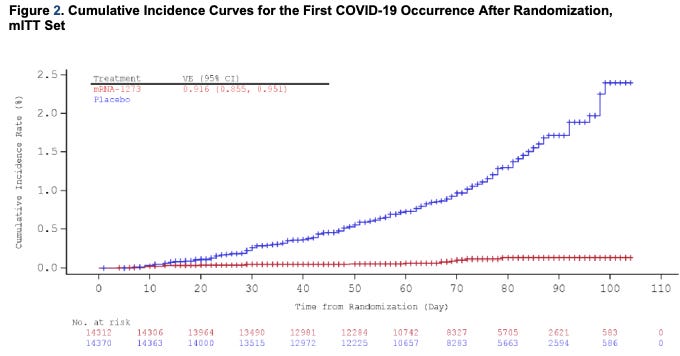
Let’s look at the Kaplan Meier curves. [As a reminder, Kaplan Meier curves graph incidence of an outcome over time, with the horizontal axis representing time and the vertical axis representing a positive COVID test.]
Just as with the Pfizer vaccine, you see a really interesting thing happening if you look at the curves in detail.
You’ll see that the two curves start to separate out around day 14 here. This means that you start to see efficacy of the vaccine 2 weeks before the second dose is given.
The briefing document estimates that after day 14, the vaccine is about 92% effective in patients who only received one dose. That’s not what the study was designed to test, so I would be cautious about extrapolating this result. It certainly doesn’t mean that 1 dose is enough, but it’s still exciting news.
There were no cases of severe COVID in the group that got both doses of the vaccine (although there were 2 severe cases in the vaccine group 2 after dose 1).
There was no signal for greater or lower efficacy in any subgroup of the trial population.
All in all, the Moderna vaccine looks like it works pretty fantastically.
What about asymptomatic infection?
In a separate FDA document, there’s actually some data on this on the Moderna vaccine. I couldn’t find any for the Pfizer vaccine in any of their documents, so I don’t know how this would compare.
The investigators did nasal swabs for participants before dose 1 and before dose 2 and found significant protection of the first dose against asymptomatic infection.
If you look at the two groups (placebo and vaccine) and exclude people who had symptomatic infection, they found 14 asymptomatic infections in the vaccine group and 38 asymptomatic infections in the placebo group.
This suggests that some asymptomatic infections are prevented after only 1 dose. We don’t have any more detailed information here, and we certainly don’t have anything long term.
How safe is this vaccine?
I wrote in my newsletter on the Pfizer vaccine about the types of adverse reactions that we should expect from this vaccine:
“There are two groups of side effects that we can expect as normal with any vaccine. First is a local reaction, i.e. a reaction to the injection itself. Redness, itching, pain, etc.
The second expected side effect is due to our body mounting an immune response to the vaccine. We would expect a certain proportion of people to get a fever and flu-like symptoms. This should be transient, lasting for about a day, and is not “getting the flu from a flu shot” but instead is a normal part of the process of generating immunity from vaccination.”
I don’t think it’s worth spending that much time on these reactions. Having some soreness in an area where you got an injection is to be expected. And since your body is mounting an immune response with vaccination, the short term fever and mild flu-like symptoms doesn’t make me worried either.
It’s not to say that these can’t be a big deal if you get them, I just don’t consider them evidence of a vaccine’s “danger,” and that danger is really what we’re looking for in the safety data.
OK, enough on the background, get into the actual safety issues
About 10% of people had some lymph node swelling in the armpit area that could persist for up to a week. This was more likely after the second shot than the first.
Fatigue, headache, transient joint pain, and muscle aches were seen in about two thirds of the vaccine group and about a third of the placebo group.
About 15% in the vaccine group had a fever, with similar numbers after the first and second shot.
Between 1-2% of patients had some type of allergic reaction to the vaccine, but about 1% of the placebo group experienced an allergic reaction as well.
There were 3 cases of Bell’s palsy in the vaccine group and 1 in the placebo group.
The death information is worth reading in full, so I’ll quote a part that drew my attention (emphasis mine):
“As of December 3, 2020, 13 deaths were reported (6 vaccine, 7 placebo). Two deaths in the vaccine group were in participants >75 years of age with pre-existing cardiac disease; one participant died of cardiopulmonary arrest 21 days after dose 1, and one participant died of myocardial infarction 45 days after dose 2. Another two vaccine recipients were found deceased at home, and the cause of these deaths is uncertain.
One patient in the placebo group died of COVID.
Now, before you overanalyze the part of that quote above that I bolded, you should know that there isn’t a signal for something nefarious happening. It’s unlikely to be related to the vaccine, but there isn’t an explanation so I made note of it.
There were a 6 pregnant patients who received the vaccine and 7 who received the placebo. Pregnancy outcomes were unknown. The one miscarriage that occurred happened in the placebo group.
So which one should I get?
You should get the one that’s offered to you. I doubt most people will have a choice. I would be fine getting either one of these options.
I personally do not have a preference, although it might be nice to get the one that’s 21 days apart (Pfizer) instead of 28 days apart. Although the one that’s 28 days apart (Moderna) doesn’t need to be stored at temperatures as low, so it may be easier to distribute without interruption of temperature control.
I doubt that there’s much difference in real world effectiveness between the two.
The data for both of these vaccines are just super promising in terms of effectiveness and overall quite reassuring when it comes to safety.
I’ve said this before and I’ll say it again: you should be expecting some weird adverse reactions to be noted as vaccines get more widely distributed. We will all feel like we know a lot more once a few million doses are out there for a couple of months.
We do not know what the future will bring, but you can be confident that there will be reports of adverse reactions to these vaccines.
TL;DR: I’ve read the papers and the FDA briefings for both vaccines. I’m confident enough in the effectiveness and safety to get either one.
Thank you for reading! If you’re enjoying my newsletter, please consider sharing with your friends and family and encouraging them to subscribe!
I always appreciate any feedback or thoughts you might have. You can reply directly to this email to reach me directly.