You may have just heard that FDA approved a drug called Aduhelm (the generic name is aducanumab) for the treatment of Alzheimer’s disease via their accelerated approval pathway.
In the news, I’ve been reading that the approval of aducanumab is a controversy, with “fierce debate over whether it works.”
I don’t think it’s actually a controversy. It’s a scandal.
I’m appalled about this decision, and you should be too.
This approval decision is terrible for patients and for doctors, and it’s a quintessential example of why so many people don’t trust the pharmaceutical industry, the FDA, and many in the healthcare field.
The drug is expected to cost $56,000 per year, and the CEO of Biogen, the company that makes Aduhelm, promises not to raise the price for about 4 years.
I find everything about this to be bonkers.
Think about this: Biogen had previously announced in 2019 that they weren’t seeking FDA approval for the drug and had discontinued the clinical trials looking into its efficacy because of lack of benefit.
This will be bad for people with Alzheimer’s right now, bad for clinical trials in the future (and not just in Alzheimer’s disease), bad for the healthcare system, and may even cost *you* more money for your own healthcare.
Let’s start with the obvious - the drug kind of stinks
There is a reason that Biogen couldn’t show an initial benefit in the two clinical trials they designed to test this medication - it doesn’t seem to really do that much with regards to stopping Alzheimer’s progression.
This drug does not reverse Alzheimer’s. The goal of aducanumab is to slow progression of disease.
And aducanumab doesn’t do this all that well (if it actually does so at all).
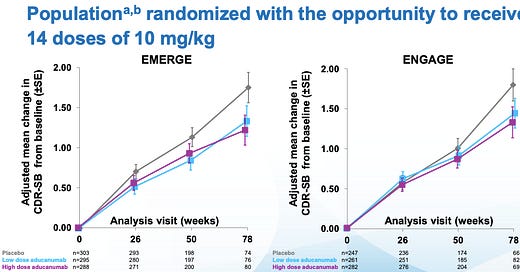
Biogen has a slide deck available showing topline results from the two trials - ENGAGE and EMERGE.
They studied patients who had mild cognitive decline who had MRIs demonstrating they had deposits of amyloid plaques (more on these below) in their brains. They randomized patients to aducanumab or placebo and monitored serial MRIs of their brains and their mental status exams.
The drug was fairly well tolerated, but a significant proportion of patients developed evidence of brain swelling from the high dose of the drug, which also happens to be the dose where benefit seems to be seen.
ENGAGE was a neutral trial - it didn’t show any benefit between aducanumab and placebo. But if you take out the subgroup from this trial that received the highest dose, you saw a small benefit. [Note: subgroup analysis can’t draw full conclusions, it is used for hypothesis generation, not definitive answers]
EMERGE only showed a small benefit.
Look at the curves here:
So the most generous view of the effectiveness of aducanumab is that is has a tiny impact on slowing the rate of worsening in Alzheimer’s in a small subgroup of patients. This isn’t a cure, and it certainly isn’t a game-changing treatment.
Take a step back - even the proposed mechanism of benefit from this drug may be wrong
Aducanumab is a drug that targets abnormally folded proteins that have deposited in the brain, which are found in patients with Alzheimer’s disease.
We’ve known that patients with Alzheimer’s have abnormal collections of these proteins in their brains since Alois Alzheimer first looked under a microscope at the brain of a patient with dementia in the early 1900s.
The “amyloid hypothesis” posits that these protein collections are the cause of cognitive decline in patients with this disease.
But there’s considerable disagreement in the world of neurology about the amyloid hypothesis of Alzheimer’s disease. We don’t really know whether these tangles are the cause of Alzheimer’s or whether they’re just an effect of some other abnormality.
The numerous negative trials looking at drugs targeting these plaques preceding this approval point away from the amyloid hypothesis. As does the fact that amyloid buildup in the brain doesn’t always correspond to the presence or severity of dementia.
And many leaders in the field no longer believe the amyloid hypothesis to be convincing.
The FDA screwed up - the bar for approval needs to be way higher
If you approve drugs that barely change the course of disease, that’s what the market is going to offer.
Permitting crappy endpoints to drive approval leads to more trials testing crappy endpoints.
There’s already an ongoing catastrophe in cancer treatments, catalogued by Vinay Prasad in his podcast Plenary Session and his book Malignant, where we spend tons of money on cancer treatments that barely impact the course of the disease because the FDA is willing to approve drugs based on mediocre studies.
The FDA doesn’t set the bar for approval at a drug that alters the course of disease in a meaningful way. So why should a drug company try to achieve something that’s more expensive at greater risk when they can get a drug like aducanumab approved?
I want to take a step back here and note what a remarkable achievement this drug is. Aducanumab is an incredible creation. Really!
Think about this - we found abnormal proteins in the brains of patients with dementia and then Biogen created a drug that gets rid of these proteins. It’s pretty remarkable!
Of course a medication like this is going to be expensive. Think about how amazing it is that it does what it does.
But just because it’s a remarkable scientific achievement doesn’t mean a drug like this should be approved or prescribed.
You should be upset! These decisions cost you money!
Health care costs are insane in our country. And essentially no patient who receives a drug like this pays for its full cost.
The cost of this $56,000 treatment that doesn’t even make much impact in the course of someone’s disease gets put into your healthcare premiums and your tax dollars.
When drugs like this are approved and prescribed, the only ones who end up better off are the people who make the drug.
If a drug for cancer extends survival by a few months but costs tens (or hundreds) of thousands of dollars, you end up getting the bill.
I am not arguing that anyone should be prohibited from receiving expensive drugs - but I am arguing that we shouldn’t all have to pay for it with our health care premiums and our tax dollars.
Approval decisions like this are why people think health care is a racket and the government is looking out for corporate interests and not the people.
Expensive drugs aren’t the place to spend money in dementia
The upcoming cost bomb of Alzheimer’s has the potential to be a medical, social, and financial disaster for many people, families, and budgets.
Some estimates expect that dementia will cost us $20 trillion over the next 40 years.
And every case of dementia is a tragedy for the patient and family involved. I am not minimizing how terrible this disease is.
So I fully understand why there’s an eagerness from the FDA to approve a treatment like this - they see what’s coming down the road and are trying to help.
But the money that would be spent on aducanumab is better spent on so many other things.
What about Alzheimer’s prevention? The vascular and metabolic contributions to dementia are becoming increasingly recognized. The same treatments that reduce the risk of heart disease - blood pressure, blood sugar/diabetes, cholesterol - all reduce the risk of dementia. Plus they’re cheaper and safer.
What about more home health aides? For the cost of one year of this drug, we could hire a home health aide to work with an advanced dementia patient on a daily basis.
Taking a drug - or prescribing one - that doesn’t do much to alter the course of this disease but costs a huge amount of money is a misuse of limited resources.
When it comes to healthcare and policy, you can’t have everything. Resources are finite, so we need to make better choices.
A more rigorous approval process that incentivizes achieving outcomes that matter would be a good place to start.
Thank you for reading! Please share on social media and encourage your friends and family to subscribe!